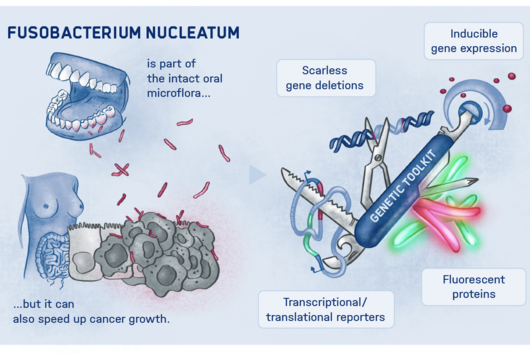
Fusobacteria and Cancer
New study on Fusobacterium nucleatum: Why does the oral cavity germ colonize and accelerate tumors?
Würzburg, November 3, 2022—Scientists at the Helmholtz Institute for RNA-based Infection Research (HIRI) and the Institute for Molecular Infection Biology (IMIB) in Würzburg want to better understand how the oral cavity germ Fusobacterium nucleatum is exactly linked to various cancer diseases. To unravel the molecular strategies of these bacteria, the team has developed new genetic tools. They discovered an adaptation factor that may help the microorganisms colonize tumor cells. The findings contribute to the search for new therapeutic targets and were published recently in the journal PNAS (The Proceedings of the National Academy of Sciences).
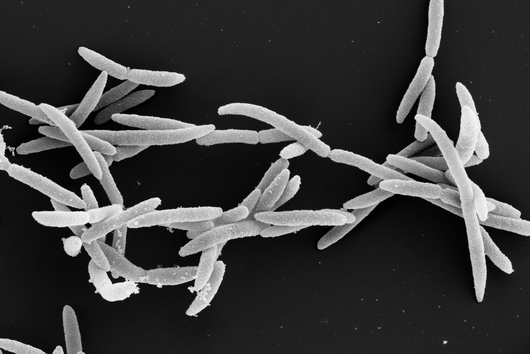
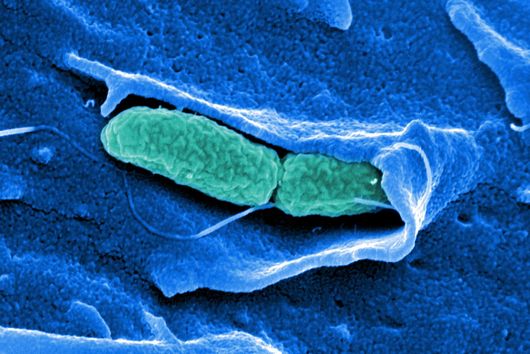
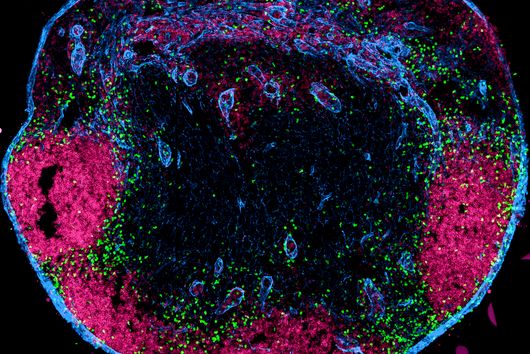
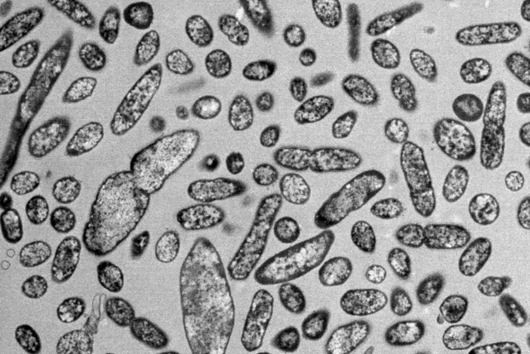
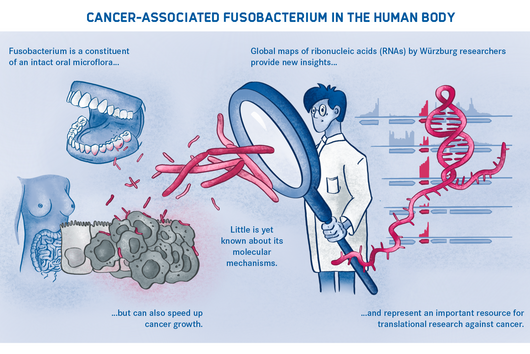
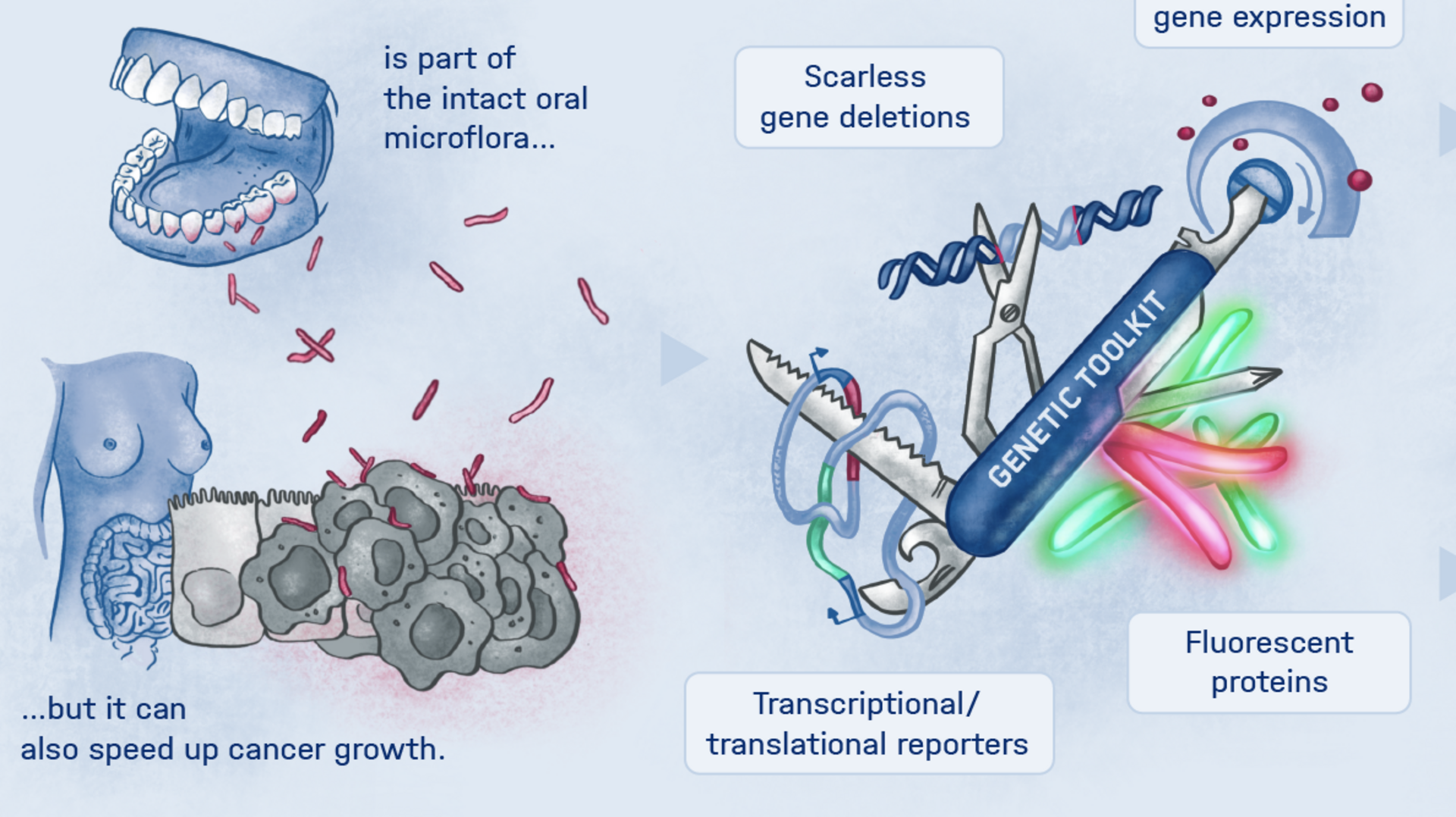
More than 4,500 species of bacteria colonize humans. Although their importance for well-being and health as well as for diseases is increasingly appreciated, the underlying molecular mechanisms are still largely unknown. This also applies to Fusobacteria: They are an abundant member of the oral microbiome, but can spread throughout the body and colonize secondary sites, especially cancer tissues. There, they promote tumor progression and metastases, complicate treatment and worsen the diagnosis. This has already been demonstrated in colorectal and breast cancer. In addition, Fusobacteria are more and more considered to play a corresponding role in cancers of other organs, such as the esophagus and pancreas.
Yet how does the oral cavity germ manage to adapt and survive beyond its original habitat? Answering this question may lead to new therapeutic approaches in the fight against cancer—and is therefore an important concern of Jörg Vogel, Managing Director of the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg and corresponding author of the current publication. His institution is a site of the Braunschweig Helmholtz Centre for Infection Research (HZI) in cooperation with the Julius-Maximilians-Universität of Würzburg (JMU), which includes the Institute for Molecular Infection Biology (IMIB) involved in the study.
"Fusobacteria are clinically of high relevance, but little is known about gene regulation in the microbes themselves," Jörg Vogel states. "One goal of my research groups at HIRI and IMIB is to understand the functions of these microorganisms at the molecular level." From there, the scientists want to identify new strategies for therapeutic and diagnostic approaches, the professor explains.
On the track of an adaptation specialist
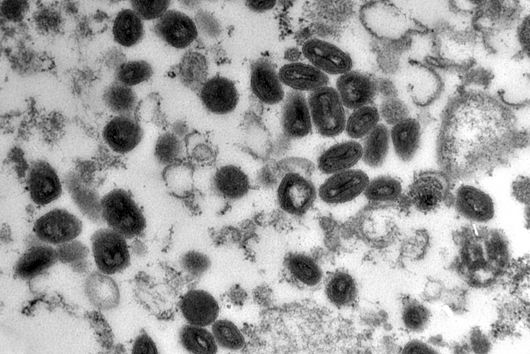
Fusobacterium nucleatum is a bacterial strain that has distanced itself early on from other known bacteria such as Escherichia coli (E. coli) in the course of evolution. Consequently, insights in the well-studied model organism E. coli may not necessarily be presumed for the oral germ. Likewise, new genetic tools are required to unravel the mystery of Fusobacteria. While lacking the ability to genetically dissect the molecular principles of the germ, research to date has primarily focused on the host.
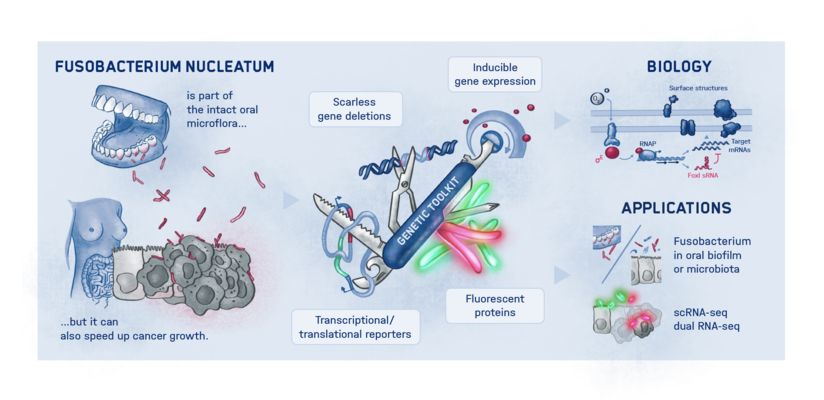
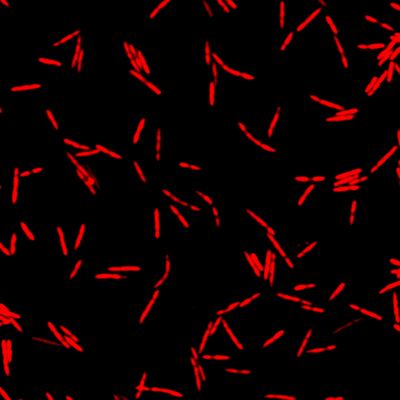
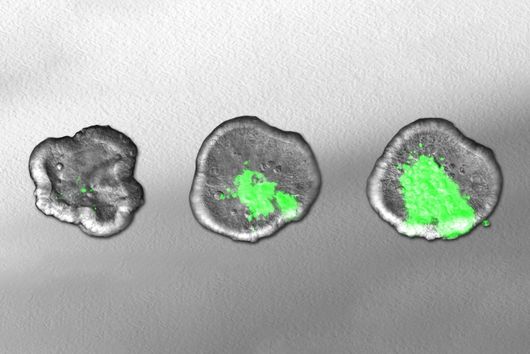
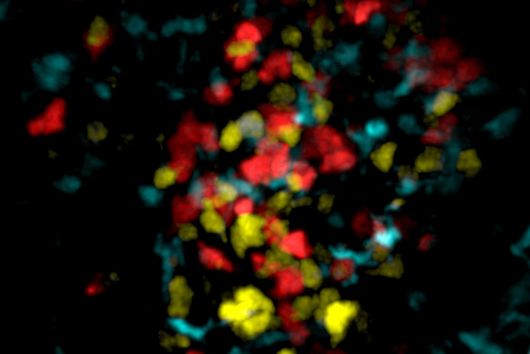
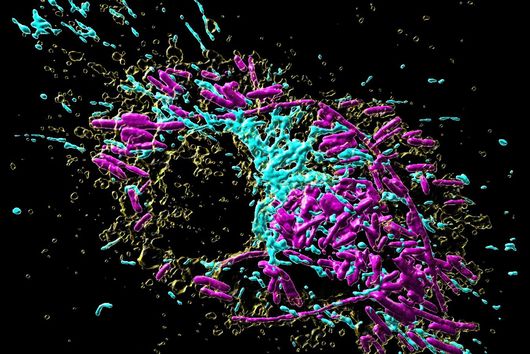
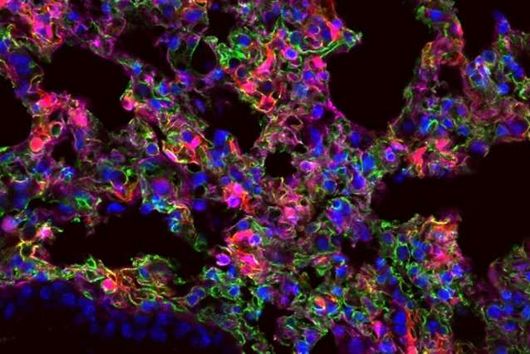
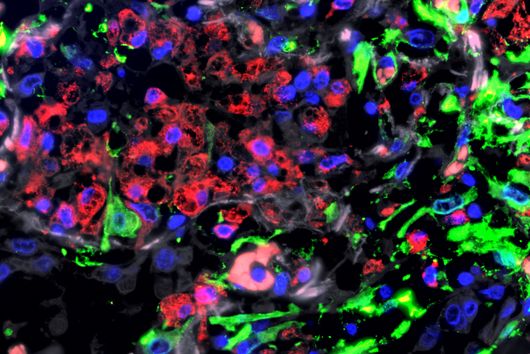
This is where the Würzburg scientists come in. "We have developed and applied a broad suite of tools for Fusobacterium nucleatum, including fluorescence imaging that allows us to visualize and track the microorganisms," explains Falk Ponath, first author of the study published in the journal PNAS (The Proceedings of the National Academy of Sciences). Using these tools, the team discovered a factor that may contribute to the adhesion of the oncomicrobes to tumor cells.
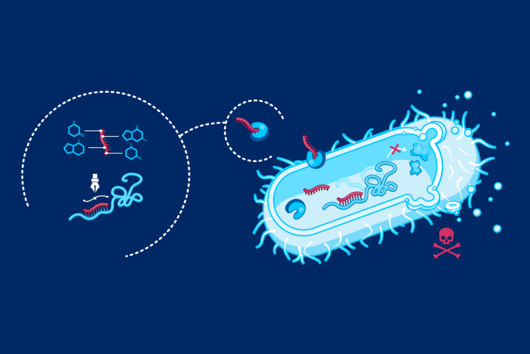
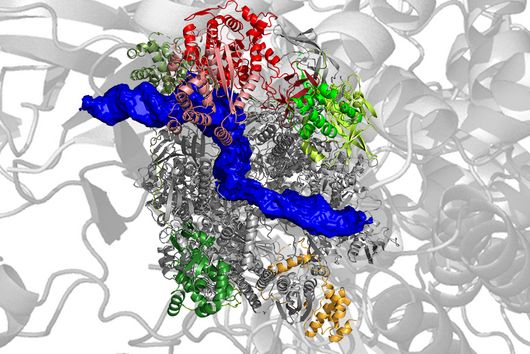
Ponath: "In a previous study, we had already seen that a small regulatory ribonucleic acid, known as sRNA, regulates a protein of the bacterial envelope. Now we have analyzed this mechanism in more detail and found a specific adaptation factor involved, which suppresses several membrane proteins." This adaptation factor was shown to be insensitive to external stressors, but strongly responded to molecular oxygen. Oxygen activated the adaptation factor, which in turn turned on the sRNA.
To discover a regulon of similar architecture to E. coli in Fusobacterium nucleatum is quite surprising in view of the evolutionary distance, says Ponath. At the same time, he adds, it is tempting to speculate that the adaptation factor serves the role of an environmental sensor and, mediated by oxygen, remodels the bacterial envelope.
Fusobacteria use the proteins of their cell membrane to interact with the host. However, evidence for a causal relationship between the aformentioned processes and the colonization of tumor tissue remains to be demonstrated. The current findings and the new genetic tools are expected to accelerate further scientific research in this area.
Funding
Falk Ponath, first author of the study, and the author Valentina Cosi were funded by the fellowship program of the Würzburg Vogel Foundation Dr. Eckernkamp. The study was further supported by funds from the Gottfried Wilhelm Leibniz Award of the German Research Foundation (DFG) to Jörg Vogel and the Rbiotics project in the Bavarian research network bayresq.net.
Original publication
Ponath F, Zhu Y, Cosi V, Vogel J (2022)
Expanding the genetic toolkit helps dissect a global stress response in the early-branching species Fusobacterium nucleatum.
The Proceedings of the National Academy of Sciences (PNAS), DOI: 10.1073/pnas.2201460119
Helmholtz Institute for RNA-based Infection Research
The Helmholtz Institute for RNA-based Infection Research (HIRI) is the first institution of its kind worldwide to combine ribonucleic acid (RNA) research with infection biology. Based on novel findings from its strong basic research program, the institute’s long-term goal is to develop innovative therapeutic approaches to better diagnose and treat human infections.
HIRI is a site of the Braunschweig Helmholtz Centre for Infection Research (HZI) in cooperation with the Julius-Maximilians-Universität Würzburg (JMU) and is located on the Würzburg Medical Campus.

Press contact