Silver linings in the pandemic
Protein ZAP inhibits multiplication of SARS-CoV-2 by 20-fold / Study published in "Nature Communications"
Würzburg / Braunschweig, Germany, December 10, 2021 – Scientists at the Würzburg Helmholtz Institute for RNA-based Infection Research (HIRI) and the Helmholtz Centre for Infection Research (HZI) in Braunschweig demonstrate for the first time how ZAP, a protein of the human immune defense system, inhibits the replication mechanism of the SARS-CoV-2 coronavirus and can reduce the viral load by 20-fold. The findings were published today in the journal Nature Communications. They may help develop antiviral agents in the fight against the pandemic.
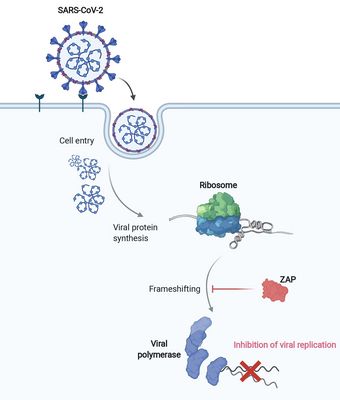
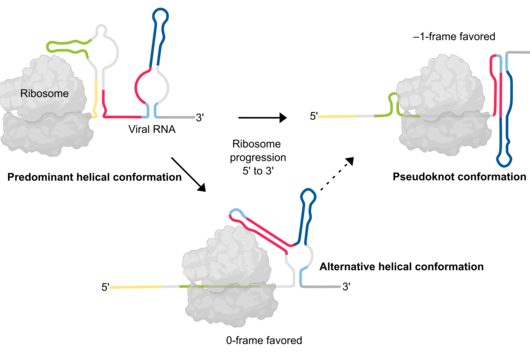
SARS-CoV-2 and other viruses whose genetic material consists of ribonucleic acids (RNA) use a propagation trick called programmed ribosomal reading frameshift. In doing so, these viruses prove to be masters of manipulation: they invade host cells and hijack the process the cells use to read the genetic information from a messenger RNA and produce proteins. The viruses alter the reading frame: this allows them to produce their own proteins in order to multiply.
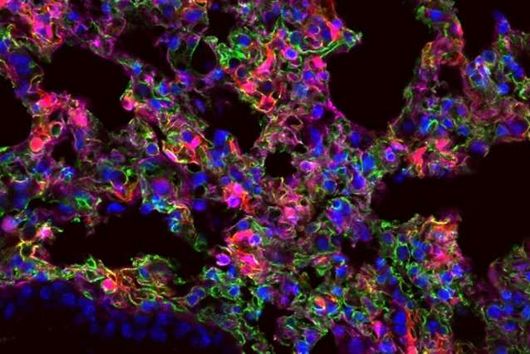
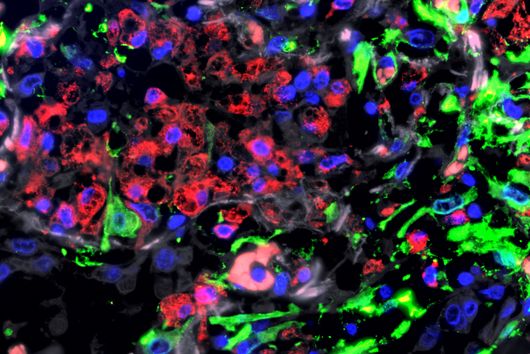
In the search for ways to stop this propagation trick in the SARS-CoV-2 coronavirus, researchers at HIRI have now identified a restriction factor called ZAP. ZAP (Zinc Finger Antiviral Protein) is already known as an immunomodulatory and antiviral protein. "ZAP is a multifunctional molecule in the immune response that can calm an exuberant immune response and shut down viral activity," explains Neva Caliskan, research group leader at HIRI and principal investigator of the study.
Sharp drop in viral load
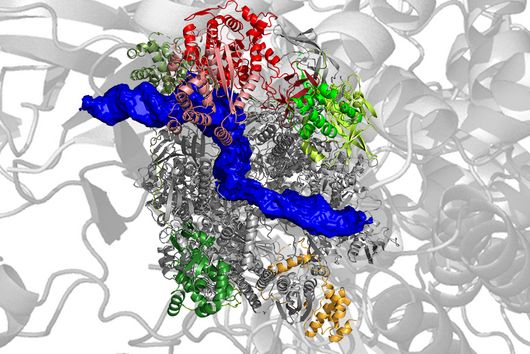
Until now, it had not been explored whether and how proteins such as ZAP interfere with the ribosomal frameshift of SARS-CoV-2. "The frameshift event has evolved as a hallmark of viral replication. This makes it a very attractive drug target," said Matthias Zimmer, one of the two first authors of the study. "Interestingly, we were able to demonstrate that ZAP binds to the viral RNA that triggers the frameshift," adds the HIRI PhD student from Caliskan's research group.
"ZAP interferes with the structural folding of coronavirus RNA and disrupts the signal that SARS-CoV-2 sends to induce host cells to produce its replication enzymes," says HIRI PhD student Anuja Kibe, second first author of the study, describing the protein's antiviral effect. And what's more: in collaboration with researchers at the HZI, the team was able to demonstrate that host cells with elevated levels of ZAP show an approximately 20-fold reduction in the amount of virus they produce. The presence – or absence – of the protein could thus also be an indicator of whether a corona infection takes a mild or severe course.
More research is needed to fully understand the molecular mechanisms behind this. However, the study results are already extremely promising: "Our findings give us hope that ZAP could be used as a template to develop potential new antiviral agents," says Caliskan.
About ZAP in the current study
Zinc Finger Antiviral Protein (ZAP for short) is a multifunctional immune defense protein that inhibits the replication of certain viruses. It exists in a short (ZAP-S) and a long form (ZAP-L). The effects described in the current study refer to ZAP-S.
About the HIRI
The Helmholtz Institute for RNA-based Infection Research (HIRI) is the first institution worldwide to combine ribonucleic acid (RNA) research with infection biology. Based on novel findings from its strong basic research program, the institute's long-term goal is to develop innovative therapeutic approaches to better diagnose and treat human infections.
HIRI is a location of the Helmholtz Centre for Infection Research (HZI) in Braunschweig in cooperation with the Julius-Maximilians-Universität Würzburg (JMU) and is located on the Würzburg Medical Campus.
Publication
Zimmer M, Kibe A, Rand U, Pekarek L, Ye L, Buck S, Smyth R, Cicin-Sain L, Caliskan N. The short isoform of the host antiviral protein ZAP acts as an inhibitor of SARS-CoV-2 programmed ribosomal frameshifting. Nature Communications, Dec. 10, 2021 DOI: 10.1038/s41467-021-27431-0.
The study was supported by funding from the Helmholtz Association, the Lower Saxony Ministry of Science and Culture, and the European Research Council.

Press contact